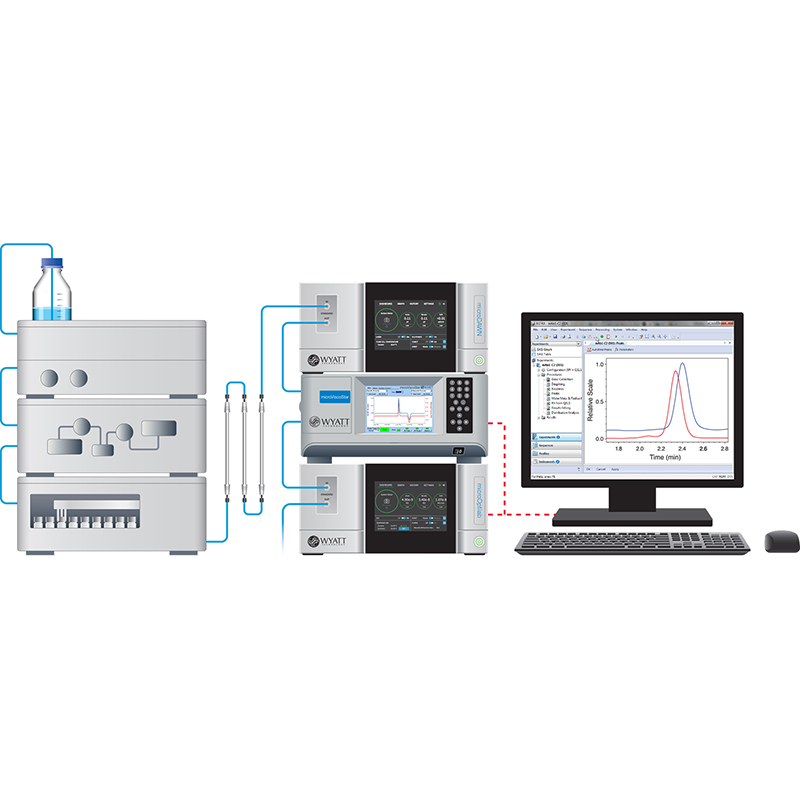
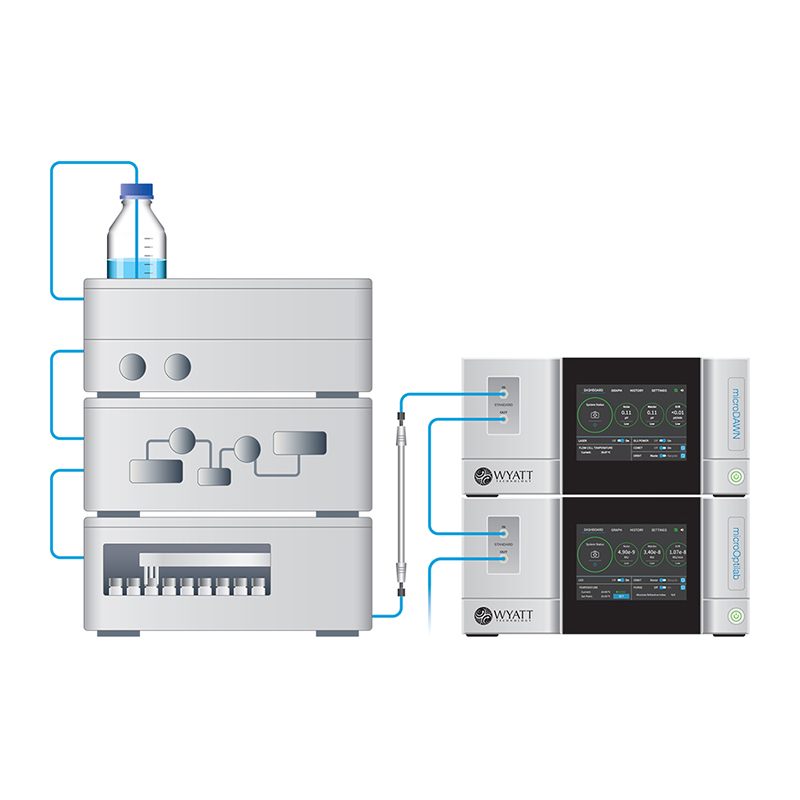
Description
Benefits of RT-MALS
- Accelerate process development and scale-up
- Increase yield, reduce overall time until lot release
- Maintain product quality with confidence
Where MALS and PAT meet
The ultraDAWN is a unique member of the DAWN family of MALS instruments. Instead of residing in the analytical lab like Wyatt’s other MALS instruments, it is adapted to pilot plants and production environments. OBSERVER software is used to calculate and report molar mass and size, 30 times per minute, as opposed to ASTRA’s post-separation, detailed off-line analysis and reporting.
PAT instrumentation is well-known and typically monitors process parameters such as pH, temperature, pressure and feedstock, which are tied indirectly to product properties and quality. UltraDAWN revolutionizes process development with its continuous, direct measurements of actual product properties.
With an intelligent user interface indicating molar mass and size alongside system health diagnostics, photodetectors at eighteen scattering angles plus one more to measure light transmitted through the flow cell, the ultraDAWN provides a solution for:
- Real-time monitoring of Mw from 103 to 109 g/mol and Rg from 10 to 250 nm. For molar mass analysis, the current version of OBSERVER™ software requires fixed analyte concentration and solvent composition
- Immediate detection of particulate contamination
- Triggering process endpoints or critical alarms
Process integration options
- In-line or on-line configuration
- Process or slipstream flow rates from 0.1 mL/min to 5 mL/min
- Integration with production control software via OPC
Real-Time Operation
In-line – For continuous processes that take place at flows below 5 mL/min, such as microfluidic drug encapsulation or chromatographic purification steps, in-line RT-MALS provides the shortest feedback loop while the ultraDAWN samples the entire process stream.
On-line
When processing takes place within a fixed volume such as a homogenizer, or when the process flow is greater than 5 mL/min, a bypass stream is diverted from the manufacturing process to the ultraDAWN. RT-MALS data are produced in near-real-time.
At-line / Off-line
For detailed analysis, products can be characterized by techniques such as SEC-MALS or FFF-MALS. These provide complete, high-resolution distributions of molar mass and size, though not in real time. A standard DAWN® MALS instrument is employed along with an SEC system or an Eclipse™ field-flow fractionation system.
RT-MALS for PAT
A PAT strategy that incorporates ultraDAWN for RT-MALS sees reduced development time and fewer samples sent to off-line analytical labs. With real-time CQA analysis, process margins can be optimized and attribute drifts detected immediately, reducing discarded product and increasing yield.
RT-MALS in Lab R&D
RT-MALS can be implemented early in process development – at pilot plant scales and even smaller, such as regular production of small batches of material for R&D use. Experience with lab-scale RT-MALS paves the way for effective implementation on the production floor.
RT-MALS in Scale-up from Lab to Manufacturing
Scaling up often means re-developing the process, requiring off-line analysis of numerous samples collected from intermediate and final steps. RT-MALS greatly accelerates scale-up: it provides real-time feedback, indicating the effect of method tweaks on CQAs, while simultaneously reducing effort and the number of samples analyzed off-line.
Unlike complex at-line PAT devices, ultraDAWN can simply be left in the process flow upon completion of scale up, for continuous or periodic monitoring of the final process.
RT-MALS on the Manufacturing Floor
ultraDAWN and OBSERVER software are simple, robust technologies that may be employed in different ways during serial production. During initial stages, technicians can visually monitor molar mass and size, intervening in the process as needed. With increasing confidence in the capabilities and reliability of RT-MALS, engineers may implement fully-automated process control using OBSERVER’s configurable triggers. In both cases, all data can be reviewed post-production to determine if any deviations were encountered.